Chapter I General Provisions
Article 1 The Provisions are formulated with the view to strengthening medical device standard work and ensuring the safety and effectiveness of medical devices in accordance with the Regulations for the Supervision and Administration of Medical Devices.
Article 2 All organizations or individuals engaged in the development, production, distribution, use, supervision and administration of medical devices within the territory of China shall comply with the Provisions.
Article 3 Medical device standards encompass national standards, industry standards and registration product standards.
1. The national or industry standards refer to those standards of which the technical requirements of the products shall be standardized nationwide;
2. The registration product standards refer to those standards set by manufacturers to ensure safety and effectiveness of the products; and upon application for product registration, they shall be reviewed by the drug regulatory departments at the level of municipalities consisting of districts or above, in accordance with relevant requirements in the national and industry standards.
Article 4 The State shall adopt an incentive system for the medical device standard work.
Chapter II Regulatory Authorities for the Standard Work and Their Functions
Article 5 The drug regulatory department under the State Council performs the following functions:
1. to organize the implementation of laws and regulations concerning medical devices, and to formulate principles, policies and provisions concerning the work of medical device standards;
2. to organize the formulation and implementation of programs and plans concerning the work of medical device standards; to guide and supervise the work of medical device standards nationwide;
3. to organize the drafting of the national standards of medical devices; to organize the formulation and promulgation of the industry standards of medical devices; to review the registration product standards of the import medical devices and of Class III medical devices made in China, in accordance with relevant requirements in the national and industry standards;
4. to supervise the implementation of the medical device standards;
5. to oversee technical committees on standardization of medical devices in various specialties;
6. to organize the conversion of the international standards and to carry out international exchanges of standards;
7. to be responsible for commending and rewarding in the work of standards; to manage funds for the work of standards.
Article 6 The drug regulatory department under the State Council establishes a technical committee on medical device standardization. The technical committee shall provide technical guidance and coordination for the work of medical device standards nationwide and perform the following functions:
1. to conduct research on the medical device standard system and to submit proposals with respect to the policies on the work of medical device standards and the program for projects of standards;
2. entrusted by the drug regulatory department under the State Council, to examine the national and industry standards of medical devices and to review the registration product standards of import medical devices and Class III medical devices made in China;
3. to guide and coordinate the work of technical committees on standardization of medical devices in every specialty;
4. to carry out training, publicity, technical guidance about the work of standards as well as academic exchanges of standards home and abroad;
5. to circulate information about the medical device standard work.
Article 7 The assignments of technical committees on standardization of medical devices in every specialty established by the State:
1. to publicize and implement laws, regulations, principles and policies regarding the work of standards;
2. to submit proposals for the programs and plans with respect to the establishment, revision and research project of the national or industry standards of medical devices in every specialty; and to conduct research on medical device standards;
3. to formulate and revise the national and industry standards and to collate, check and edit the standards to be submitted for approval;
4. to provide technical guidance on the work of medical device standards; to assist the drug regulatory authorities at various levels to solve technical problems arising in the implementation of standards;
5. to collect and collate materials relating to medical device standards and to establish the technical files of the medical device standards in the specialty concerned;
6. to promote publicity and implementation of the national and industry standards of medical devices and to develop academic exchanges in the field; to assist in the training of the personnel engaged in the work of standards.
Article 8 The drug regulatory authorities of provinces, autonomous regions, and municipalities directly under the Central Government shall fulfill the following duties within their respective jurisdiction:
1. to implement laws, regulations, principles and policies concerning the work of medical device standards;
2. to supervise the implementation of medical device standards within their jurisdiction;
3. to re-examine the registration product standards of the medical devices produced within jurisdiction; to conduct the preliminary examination of the registration product standards for Class III medical devices made within their jurisdiction;
4. to guide and coordinate the drafting of the national and industry standards as entrusted.
Article 9 The drug regulatory authorities of municipalities consisting of districts are responsible for reviewing the registration product standards for Class I medical devices within their jurisdiction. The drug regulatory authorities of municipalities consisting of districts or counties (cities at county level) are responsible for supervising and examining the implementation of the medical device standards within their jurisdiction.
Chapter III Establishment and Issuance of National and Industry Standards
Article 10 The organization drafting the standards shall conduct scientific verification and technical analysis of the requirements, experimental methods and testing procedures of the standards and properly summarize the verification; they shall prepare the draft version of the standards and the description on compiling the standards and other relevant appendices as required.
Article 11 The establishment and examination of the national and industry standards for medical devices shall be organized by the technical committee on medical device standardization under the drug regulatory department of the State Council or by technical committees on standardization of medical devices in every specialty which are established by the State.
Article 12 The approved standards shall be modified by the drafting organization and, after being reviewed by the secretariat of the corresponding technical committee on standardization, shall be submitted to the drug regulatory department under the State Council. The industry standards shall be approved, numbered and issued by the drug regulatory department under the State Council.
Chapter IV Establishment and Examination of the Registration Product Standards
Article 13 The registration product standards shall be established in line with the national and industry standards as well as relevant laws and regulations and shall be drafted as required in the Norms for Compiling the Registration Product Standards of Medical Devices issued by the drug regulatory department under the State Council.
Article 14 When applying for product registration, the manufacturer shall submit the text of the registration product standard and the description on compiling the standard.
The following contents shall be included in the description on the registration product standard:
1. whether the material in contact with human body has been used in clinic and whether its safety and reliability has been proved;
2. relevant standards and materials that have been quoted or referred to;
3. basis for administrative classification;
4. the product overview and basis for determining major technical items;
5. testing report ;
6. other matters in need of clarification.
Article 15 The registration product standards for import medical devices shall be reviewed by the drug regulatory department under the State Council.
The registration product standards for Class III medical devices made in China shall first be examined by the drug regulatory authorities of provinces, autonomous regions and municipalities directly under the central government and then be submitted to the drug regulatory department under the State Council for review.
The registration product standards for Class II medical devices made within China shall be reviewed by the drug regulatory authorities of provinces, autonomous regions and municipalities directly under the central government.
The registration product standards for Class I medical devices made within China shall be reviewed by the drug regulatory authorities of the municipalities consisting of districts.
Article 16 In the preliminary examination and review of the registration product standards, the following major contents shall be covered:
1. whether the standards conform to the currently valid national and industry standards and relevant laws and regulations;
2. whether the product is named in conformity to relevant requirements;
3. whether the intended functions are defined accurately;
4. the determination of testing items and the rationality of testing procedures;
5. whether the verifying methods are appropriate and the conclusions are correct.
Article 17 The registration product standards shall be collated or modified by manufacturers in accordance with the review comments and shall be numbered and filed by the reviewing drug regulatory authorities.
The number of the registration product standard consists of four parts: the code of the registration product standard, the abbreviation of the place (country) where the standard verifying authority is located, the serial number of the registration product standard and the year number.
In this context, the abbreviation of the place where the reviewing authority is located applies to the medical devices made within China, consisting of one or two Chinese characters which are short for the name of the State, province, autonomous region, municipality directly under the central government or the name of the province or autonomous region plus the municipality consisting of districts. The abbreviation of the country consists of three English letters and applies to import medical devices.
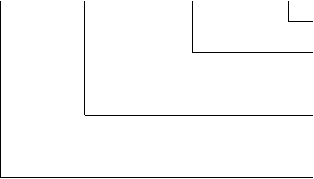 Example:
Example:
YZB /X(XXX) XXXX - XXXX
the issuing year
serial number of the registration product standard
abbreviation of the place (country) where the reviewing authority is located
code of the registration product standard
Article 18 Once the national and industry standards are amended and issued, prior to official implementation, manufacturers shall modify the registration product standards in line with the amended national and industry standards and shall fill out the Modification Form for Registration Product Standards of Medical Devices and submit it to the original reviewing authority for review.
Article 19 Manufacturers shall be responsible for the contents defined in the registration product standards.
Chapter V Implementation and Supervision
Article 20 Development, production, distribution and use of medical devices shall conform to the corresponding national standards, industry standards or registration product standards. Medical devices without corresponding standards are not allowed to be produced, distributed, and used.
Article 21 Where a manufactured medical device does not meet the registration product standard, it is considered as not conformed to the industry standard.
Article 22 The supervision and inspection personnel of the drug regulatory authorities at or above the county level shall supervise and inspect the standard implementation in organizations engaged in the production, distribution or use of medical devices. Any organizations or individuals concerned shall not refuse to provide information or conceal problems. The supervision and inspection personnel have an obligation to keep confidential the obtained materials and samples.
Chapter VI Supplementary Provisions
Article 23 The Provisions shall be interpreted by the State Food and Drug Administration of the State Council.
Article 24 The Provisions shall come into force as of May 1, 2002.
 Regulatory News
Regulatory News
 Position :
Position :